Have you ever wondered how atoms can remain on an excited state for what seems like an eternity in atomic terms? Welcome to an fascinating world of metastable states — nature’s way making “patient” excited atoms. They revolutionized laser technology and keep shaping modern physics applications.
Metastable states represent one of quantum physics’ most intriguing phenomena — excited atomic states that defy typical rush back to ground state equilibrium. Unlike ordinary excited states which release their energy within nanoseconds, metastable states can maintain their elevated energy levels for microseconds, milliseconds, or even longer periods.
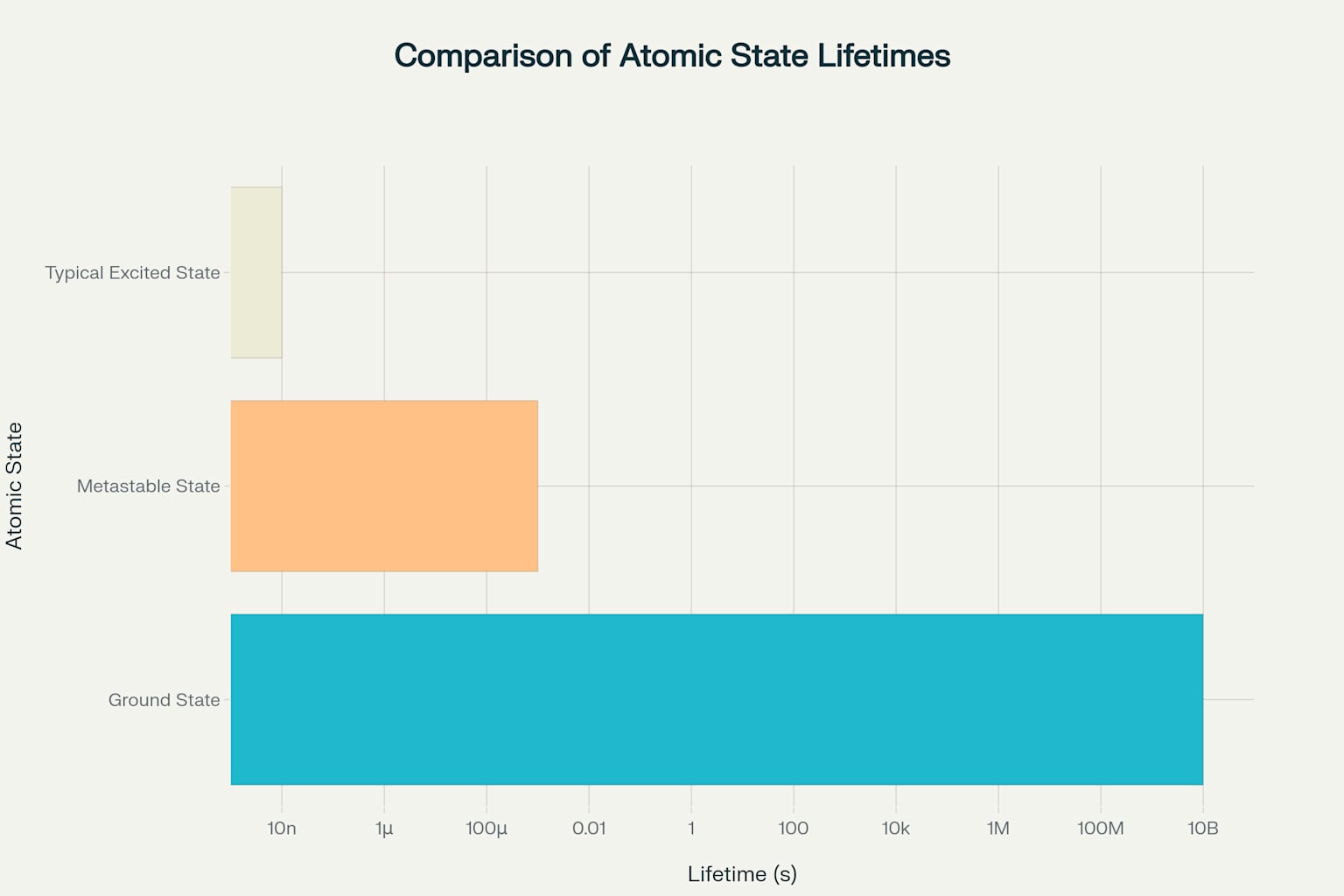
To put this in perspective, imagine an excited atom typically lasting about 10 nanoseconds before returning to its ground state. A metastable state, by contrast, can persist for times that are more than 100,000 times longer — a truly remarkable difference in atomic timescales.
The key distinction lies on transition rules governing how atoms release energy. In quantum mechanics, certain energy transitions are considered “forbidden” due to selection rules that restrict which electronic transitions can occur readily. These forbidden transitions create bottlenecks in the energy release process, effectively trapping atoms in these intermediate energy states.
The extended lifetime of metastable states results from fundamental quantum mechanical principles that govern atomic behavior. When an atom absorbs energy and becomes excited, it typically releases this energy through spontaneous emission — the process where electrons drop to lower energy levels while emitting photons.
However, metastable states exist because certain symmetries in atomic structure prohibit the dominant dipole interactions with electromagnetic fields. This means that the usual pathways for energy release are blocked or severely restricted, forcing atoms to rely on much weaker processes for transition.
These weaker processes include magnetic dipole transitions, electric quadrupole transitions, or higher-order multipole interactions. Since these alternative pathways have significantly lower probabilities of occurrence, the atom remains “stuck” in the metastable state for extended periods.
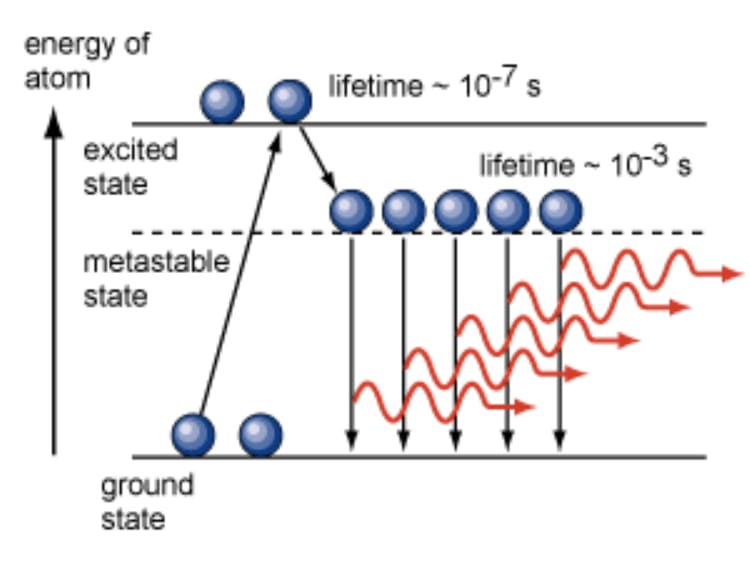
Atomic energy level diagram illustrating a metastable state with a significantly longer lifetime than a typical excited state, leading to photon emission.
The resulting lifetime comparison reveals the dramatic difference between atomic states. While typical excited states last approximately 10⁻⁸ to 10⁻⁹ seconds, metastable states can persist for 10⁻⁶ to 10⁻³ seconds — a difference spanning several orders of magnitude.
The practical importance of metastable states becomes apparent in laser technology, where they serve as the foundation for solid-state gain media. These materials depend on metastable states to achieve population inversion — a condition where more atoms exist in excited states than in ground states.

Solid-state laser gain media typically feature upper laser energy levels with metastable characteristics, allowing for the accumulation of excited atoms necessary for laser operation. The lifetimes of these upper energy levels vary significantly depending on the specific material and dopant used.
For example, erbium-doped fiber amplifiers demonstrate upper-state lifetimes of approximately 8 - 10 milliseconds, while ytterbium-doped laser gain media exhibit lifetimes of roughly 1 - 2 milliseconds. These extended lifetimes provide sufficient time for population buildup, enabling efficient laser operation.
In solid-state media, achieving long energy level lifetimes requires more than just weak spontaneous emission. The material must also minimize non-radiative transitions that could provide alternative pathways for energy loss.
Non-radiative transitions include multi-phonon processes, where vibrational energy in the crystal lattice facilitates energy transfer, and quenching effects caused by impurities that create unwanted energy dissipation pathways. Successful solid-state gain media must carefully balance these competing factors to maintain the metastable character of their upper laser levels.
Metastable states occur naturally in numerous atomic systems, with some of the most notable examples found in noble gases and rare earth elements. Neon atoms exhibit metastable states that play crucial roles in gas discharge applications, while neodymium ions in crystalline hosts form the basis for many solid-state laser systems.
One of the most dramatic examples of metastable behavior occurs in helium atoms, where the first excited state (2³S₁) holds the record as the longest-lived neutral atomic state, with a lifetime of approximately 7,870 seconds — over two hours. This extraordinary persistence demonstrates the extreme end of metastable behavior in atomic systems.
In astronomical contexts, metastable states contribute to the characteristic colors of auroras, where metastable oxygen atoms in Earth's rarefied upper atmosphere emit their stored energy as visible light after extended periods without collision. This phenomenon illustrates how environmental conditions can influence the practical manifestation of metastable behavior.
The existence of metastable states reflects deeper principles of quantum mechanics, particularly the role of selection rules in governing atomic transitions. These rules arise from conservation laws and symmetry requirements that determine which transitions are “allowed” versus “forbidden” in quantum systems.
Understanding metastable states requires appreciation for the probabilistic nature of quantum transitions, where “forbidden” doesn’t mean impossible, but rather indicates extremely low probability. This probabilistic framework explains why metastable states eventually decay, but on timescales dramatically longer than typical excited state lifetimes.
The practical applications of metastable states extend far beyond traditional laser technology. Modern quantum technologies, including quantum sensors and atomic clocks, often rely on metastable states to achieve the precision and stability required for advanced measurements.
Research continues into new materials and atomic systems that can provide even longer metastable lifetimes or novel properties for emerging applications. Understanding and controlling metastable states remains an active area of investigation in both fundamental physics and applied technology development.
Metastable states represent a fascinating intersection of fundamental quantum mechanics and practical technology applications. By providing atoms with extended excited state lifetimes, these special energy levels enable the population inversion necessary for laser operation while demonstrating the subtle but profound effects of quantum selection rules.
From the millisecond lifetimes in erbium-doped fiber amplifiers to the hours-long persistence of metastable helium atoms, these quantum phenomena continue to drive innovations in photonics, quantum technology, and precision measurement. As our understanding of atomic physics deepens, metastable states will undoubtedly continue revealing new possibilities for technological advancement and scientific discovery.
The study of metastable states serves as a perfect example of how fundamental physics concepts translate directly into transformative technologies, bridging the gap between quantum mechanical principles and everyday applications that have revolutionized modern communication, manufacturing, and scientific research.
Contact: Jason
Phone: +8613337332946
E-mail: [email protected]
Add: Hangzhou City, Zhejiang Province, China